Research Advances
Powerful Sequencing Tool Helps Identify Infectious Diseases in Mali
February 27, 2025 - A study in Bamako, Mali, used the advanced VirCapSeq-VERT sequencing tool to identify multiple viral infections in patients with unexplained fever. Conducted by the National Institute of Allergy and Infectious Diseases (NIAID), the study revealed over 40 percent of patients had coinfections, helping identify diseases like dengue, Zika, and HIV. The tool, combined with traditional methods, could improve diagnostics in resource-limited areas with high disease incidence, offering better patient outcomes.
NIAID Study Describes Successful Treatment Regimen for Person With Multidrug-Resistant HIV
January 16, 2025 - A National Institute of Allergy and Infectious Diseases (NIAID) study published in Nature Medicine describes a successful treatment regimen for a person with multidrug-resistant (MDR) HIV. The regimen included the investigational monoclonal antibody UB-421, along with antiretrovirals approved by the U.S. Food and Drug Administration (FDA), like lenacapavir. After 70 weeks of treatment, the patient’s HIV levels decreased significantly, and immune cell recovery was observed. This promising approach could offer new treatment options for those with limited alternatives due to MDR HIV.
As Prevention Strategy for Sexually Transmitted Infections (STI) Rolls Out, Experts Highlight Both Promise and Knowledge Gaps
January 6, 2025 - Doxycycline post-exposure prophylaxis has shown promise in reducing syphilis and chlamydia rates in some populations. However, it has had minimal impact on gonorrhea and may contribute to antibiotic resistance. Research highlights the need for continued monitoring. Experts also emphasize the importance of ensuring broad access and further investigation into long-term efficacy and alternative STI prevention strategies.
Researchers Investigate Potential Treatment for Eliminating HIV From the Brain
December 20, 2024 - Researchers are testing a drug called BLZ945 as a possible treatment to remove HIV from the brain. HIV can hide in certain immune cells in the brain, making it hard to treat. In a study with monkeys, BLZ945 reduced these cells and the virus in the brain without affecting other brain cells. The drug may help the immune system fight the virus. Though it's being tested for cancer, BLZ945 shows promise as a potential way to eliminate HIV from the brain.
Some People With Advanced HIV Have Anti-CD4 Autoantibodies Associated With Dampened Immune Recovery
December 18, 2024 - A analysis sponsored by the National Institute of Allergy and Infectious Diseases (NIAID) found that people with advanced HIV and anti-CD4 autoantibodies experienced limited CD4+ T-cell reconstitution for up to four years of observation after antiretroviral therapy initiation, highlighting a potential immune effect of long-term unsuppressed HIV. The findings were published in Clinical Infectious Diseases.
NIH Study Finds Tecovirimat Was Safe But Did Not Improve Mpox Resolution or Pain
December 10, 2024 - Tecovirimat was safe but did not reduce the time to lesion resolution or reduce pain among adults with mild to moderate clade II mpox and a low risk of severe disease in an international study.
NIH Trial of Rectal Microbicide for HIV Prevention Begins in the US
October 31, 2024 - A clinical trial has launched to examine the safety and acceptability of a novel rectal HIV microbicide douche containing the antiretroviral drug tenofovir. While HIV incidence is slowly decreasing in the United States, 67 percent of U.S. HIV diagnoses from 2018-2022 were among gay, bisexual, and other men who have sex with men, pointing to the need for expanded HIV prevention options.
Kidney Transplantation Between Donors and Recipients With HIV is Safe
October 16, 2024 - A study confirmed that kidney transplantation between donors and recipients who both have HIV is safe and provides similar outcomes to transplants from donors without HIV. The study, involving 198 adults with HIV and end-stage kidney disease, showed no significant differences in survival, graft survival, or rejection rates between the two groups. These findings support expanding this practice beyond research settings, addressing organ shortages and disparities in transplant access for people with HIV. The study was funded by the National Institute of Allergy and Infectious Diseases (NIAID).
HIVR4P 2024 Research Highlights: Reproductive Health While on PrEP and Signals to Guide HIV Vaccines and Cure
October 7, 2024 - New National Institute of Allergy and Infectious Diseases (NIAID)-supported science presented at the 5th HIV Research for Prevention Conference, or HIVR4P, in Lima, Peru, features a breadth of HIV discovery and translational findings and enriches the evidence base on HIV pre-exposure prophylaxis (PrEP) within the context of reproductive health.
SARS-CoV-2 Rapidly Evolves in People With Advanced HIV
September 9, 2024 - A National Institute of Allergy and Infectious Diseases (NIAID) study revealed how some SARS-CoV-2 variants could evolve. Researchers used advanced technology to examine virus genes in people with and without HIV who also had COVID-19, analyzing different virus copies over time. They found that people with advanced HIV—defined by low CD4+ T cells—had dozens of SARS-CoV-2 variants, compared to just one major variant in most people without HIV or those with HIV and higher CD4+ T cell counts.
Study Links Certain Vaginal Bacteria and Inflammatory Marker to Increased Odds of HIV Acquisition Among Women
September 9, 2024 - Fourteen vaginal bacterial species and the presence of a protein that promotes inflammation were associated with increased odds of HIV acquisition in a study of more than 500 women in African countries with high HIV incidence. The study was the largest to date to prospectively analyze the relationship between both the vaginal microbiome and vaginal tissue inflammation and the likelihood of HIV acquisition among women in this population.
Broadly Neutralizing Antibodies Evaluated in Many HIV Cure Strategies
August 6, 2024 - Many promising HIV cure strategies use broadly neutralizing antibodies (bNAbs), which neutralize a wide range of HIV variants by binding to specific viral components and triggering an immune response to destroy the virus. Several bNAbs have been developed and tested for HIV prevention and treatment. The National Institute of Allergy and Infectious Diseases (NIAID) and partners are evaluating bNAb-based strategies, alone and with other immunity-enhancing approaches, for HIV clearance in clinical trials across Africa, the Americas, and Southeast Asia.
Exploratory Analysis Associates HIV Drug Abacavir With Elevated Cardiovascular Disease Risk in Large Global Trial
July 23, 2024 - Current or previous use of the antiretroviral drug (ARV) abacavir was associated with an elevated risk of major adverse cardiovascular events (MACE) in people with HIV, according to an exploratory analysis from a large international clinical trial primarily funded by the National Institutes of Health (NIH). There was no elevated MACE risk for the other ARVs included in the analysis. The findings were presented at the 25th International AIDS Conference, or AIDS 2024, in Munich, Germany.
An Isolated Viral Load Test May Generate False Positive Results for People Using Long-Acting PrEP
July 23, 2024 - A single laboratory-based HIV viral load test used by U.S. clinicians who provide the long-acting injectable cabotegravir (CAB-LA) as HIV pre-exposure prophylaxis (PrEP) did not reliably detect HIV in a multicountry study. A single positive viral load test was frequently found to be a false positive result. However, a second viral load test with a new blood sample was able to distinguish true positive results from false positive results for all participants whose initial viral load test was positive.
Long-Acting Injectable Cabotegravir for HIV Prevention Is Safe in Pregnancy
July 23, 2024 - Long-acting injectable cabotegravir (CAB-LA) was safe and well tolerated as HIV pre-exposure prophylaxis (PrEP) before and during pregnancy in the follow-up phase of a global study among women. The analysis of outcomes from more than 300 pregnancies and infants was presented at the 25th International AIDS Conference, or AIDS 2024, in Munich, Germany.
Putting the Power of Lab-Based Diagnostic Testing in the Palm of Your Hand
July 22, 2024 - Researchers at the University of Connecticut developed a portable lab-in-a-tube device, LIAMT, which can detect HIV and SARS-CoV-2 with polymerase chain reaction (PCR) level accuracy, offering a faster, more accessible alternative to traditional lab-based PCR testing. This handheld device could revolutionize HIV diagnostics by enabling quick, reliable testing at the point of care or at home, which may improve timely access to treatment for individuals with HIV.
Youth With HIV May Have Shorter Lifespans Than Youth Without HIV, NIH-Funded Study Suggests
June 27, 2024 - Researchers developed a simulation to estimate the lifespan of youth with HIV and found that some could lose 10 to 20 years of life, compared to youth without HIV. Life expectancy depends on factors such as whether they acquired HIV around the time of birth or later in life or received ideal care.
NIH Statement on Preliminary Efficacy Results of Twice-Yearly Lenacapavir for HIV Prevention in Women
June 26, 2024 - The injectable antiretroviral drug lenacapavir was safe and 100 percent effective as long-acting HIV pre-exposure prophylaxis (PrEP) among women in a Phase 3 clinical trial, according to top-line findings released by Gilead Sciences, Inc., the study sponsor. Lenacapavir is administered every six months, making it the most durable HIV prevention method to have shown efficacy in this population.
U.S. Clinical Trials Begin for Twice-Yearly HIV Prevention Injection
June 4, 2024 - Two clinical trials have launched to examine a novel long-acting form of HIV pre-exposure prophylaxis (PrEP) in women and people who inject drugs. The midstage studies will assess the safety, acceptability, and pharmacokinetics (how a drug moves through the body) of lenacapavir, an antiretroviral drug administered by injection every six months. The studies are sponsored and funded by Gilead Sciences, Inc., and implemented through the HIV Prevention Trials Network.
Antiretroviral Drug Improves Kidney Function After Transplant in People With HIV
June 4, 2024 - An HIV drug that suppresses the activity of the CCR5 receptor—a collection of proteins on the surface of certain immune cells—was associated with better renal function in kidney transplant recipients with HIV, compared to people who took a placebo in a randomized trial. According to the authors, these findings warrant further exploration of the benefit of CCR5 antagonists in all kidney transplant recipients regardless of HIV status.
Novel Vaccine Concept Generates Immune Responses That Could Produce Multiple Types of HIV Broadly Neutralizing Antibodies
May 30, 2024 - Using a combination of cutting-edge immunologic technologies, researchers have successfully stimulated animals’ immune systems to induce rare precursor B cells of a class of HIV broadly neutralizing antibodies. The findings, published in Nature Immunology, are an encouraging, incremental step in developing a preventive HIV vaccine.
Proof-of-Concept Study Shows an HIV Vaccine Can Generate Key Antibody Response in People
May 20, 2024 - An HIV vaccine candidate elicited trace levels of HIV broadly neutralizing antibodies and high levels of other key immune cells in an early stage clinical trial. This immune response is an important signal that, if antibody levels can be further amplified, the vaccination strategy might be able to prevent HIV.
NIH Ending the HIV Epidemic Projects Bridge Gaps Between HIV Research and Public Health Practice
April 8, 2024 - The National Institutes of Health (NIH) issued $26 million in awards to HIV research institutions in its fifth year supporting implementation science under the Ending the HIV Epidemic in the U.S. (EHE) initiative. These awards are the latest investments in a program that is rapidly and rigorously generating evidence to inform the unified domestic HIV response by agencies across the U.S. Department of Health and Human Services.
Extended Care for People With HIV Improved Cardiovascular Health Outcomes
March 11, 2024 - A 12-month trial showed that an extended care program for people with HIV improved cardiovascular health outcomes. Nurses provided home support, helping participants monitor blood pressure and adjust medications. After a year, the intervention group saw significant improvements in blood pressure and cholesterol, reducing their risk of atherosclerotic heart disease by 14 percent and 9 percent, respectively. The study included 297 adults with high cholesterol and blood pressure.
Children Surpass a Year of HIV Remission After Treatment Pause
March 6, 2024 - Four children enrolled in a National Institutes of Health (NIH)-funded study remained free of detectable HIV for more than one year after their antiretroviral therapy was paused to determine if they could achieve HIV remission, researchers report. The findings suggest that very early HIV treatment enables unique features of the neonatal immune system to limit HIV reservoir development, increasing the prospect of HIV remission.
Long-Acting HIV Treatment Benefits Adults With Barriers to Daily Pill Taking and Adolescents With Suppressed HIV
March 6, 2024 - Long-acting, injectable antiretroviral therapy (ART) suppressed HIV replication better than oral ART in people who had previously experienced challenges taking daily oral regimens and was found safe in adolescents with HIV viral suppression, according to two studies presented at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver. Both studies were sponsored by the National Institute of Allergy and Infectious Diseases (NIAID) in collaboration with other National Institutes of Health (NIH) institutes.
Semaglutide Reduces Severity of Common Liver Disease in People With HIV
March 5, 2024 - A weekly injection of semaglutide was safe and reduced the amount of fat in the liver by 31 percent in people with HIV and metabolic dysfunction-associated steatotic liver disease (MASLD), according to a presentation at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver. This is the first clinical trial of semaglutide for MASLD in people with HIV.
Vaginal Ring and Oral PrEP Found Safe for HIV Prevention Throughout Pregnancy
March 5, 2024 - The monthly dapivirine vaginal ring and daily oral pre-exposure prophylaxis (PrEP) with tenofovir disoproxil fumarate and emtricitabine were each found to be safe for HIV prevention among women who started using one of them in their second trimester of pregnancy, according to findings presented at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver. Pregnant women are estimated to be three times more likely to acquire HIV through sex than peers who are not pregnant.

Tools underestimate cardiovascular event risk in people with HIV
March 4, 2024 - The elevated cardiovascular disease risk among people with HIV is even greater than predicted by a standard risk calculator in several groups, including Black people and cisgender women, according to analyses from a large international clinical trial primarily funded by NIH. The risk of having a first major cardiovascular event was also higher than previously predicted for people from high-income regions and those whose HIV replication was not suppressed below detectable levels.

New guidelines for use of statins by people with HIV to prevent cardiovascular events
February 29, 2024 - The Department of Health and Human Services Guidelines Panel for the Use of Antiretroviral Agents in Adults and Adolescents with HIV (the Panel) has developed recommendations for the use of statin therapy in people with HIV, in collaboration with representatives from the American College of Cardiology, the American Heart Association, and the HIV Medicine Association. The new guidelines, Recommendations for the Use of Statin Therapy as Primary Prevention of Atherosclerotic Cardiovascular Disease in People with HIV, were published on February 27, 2024, and can be found online at ClinicalInfo.hiv.gov
Long-acting HIV treatment demonstrates efficacy in people with challenges taking daily medicine as prescribed
February 21, 2024 - Long-acting antiretroviral therapy (ART) with cabotegravir and rilpivirine was superior in suppressing HIV replication compared to daily oral ART in people who had been unable to maintain viral suppression through an oral daily regimen, according to interim data from a randomized trial. Upon review of these findings, an independent Data and Safety Monitoring Board (DSMB) recommended halting randomization and inviting all eligible study participants to take long-acting ART.
NIH-developed HIV antibodies protect animals in proof-of-concept study: findings support the HIV fusion peptide as a promising preventive vaccine target
January 17, 2024 - Three different HIV antibodies each independently protected monkeys from acquiring simian-HIV (SHIV) in a placebo-controlled proof-of-concept study intended to inform development of a preventive HIV vaccine for people. The antibodies—a human broadly neutralizing antibody and two antibodies isolated from previously vaccinated monkeys—target the fusion peptide, a site on an HIV surface protein that helps the virus fuse with and enter cells.
Biomedical STI prevention evidence is inadequate for cisgender women
December 20, 2023 - Pivotal studies of some biomedical HIV and sexually transmitted infection (STI) prevention interventions have excluded cisgender women or demonstrated low efficacy among them, limiting their prevention options relative to other populations who experience high HIV and STI incidence. Findings from an NIH-funded study show doxycycline postexposure prophylaxis (better known as DoxyPEP) did not prevent STI acquisition in cisgender women, despite showing promising results in gay, bisexual, and other men who have sex with men and transgender women in a previous study.
NIH research identifies opportunities to improve future HIV vaccine candidates: study suggests greater CD8+ T-cell activity may increase HIV immunity
December 14, 2023 - An effective HIV vaccine may need to prompt strong responses from immune cells called CD8+ T cells to protect people from acquiring HIV, according to a new study from researchers at NIH. The study findings draw comparisons between the immune system activity of past HIV vaccine study participants and people with HIV who naturally keep the virus from replicating even in the absence of antiretroviral therapy (ART).

NIH clinical trial of tuberculous meningitis drug regimen begins: six-month multidrug regimen being evaluated against standard treatment
December 7, 2023 - A trial of a new drug regimen to treat tuberculous meningitis (TBM) has started enrolling adults and adolescents in several countries where tuberculosis (TB) is prevalent. The trial will include 330 participants aged 15 years and older who have or are likely to have TBM based on signs and symptoms, including people living with and without HIV.

Generic daily HIV prevention pill for young men who have sex with men could save lives, lower costs, NIH-funded study suggests
October 12, 2023 - Compared to annual HIV screening alone, generic daily oral HIV pre-exposure prophylaxis (PrEP) with HIV screening every three months would result in fewer HIV acquisitions, longer life expectancy, and fewer HIV-associated costs among young men who have sex with men in the United States. These predictions, which come from a simulation study supported by the National Institutes of Health, illustrate the value of promoting PrEP use in this population.

NIH-funded study finds several potential risk factors for high blood pressure disorders of pregnancy in people with HIV
September 27, 2023 - The risk for hypertensive disorders of pregnancy was higher for pregnant people with HIV if they had low CD4+ immune cell counts in the first or second trimester or if they began taking antiretroviral drug regimens after 20 weeks of pregnancy, rather than at conception, according to a study funded by the National Institutes of Health.

Clinical trial of HIV vaccine begins in United States and South Africa
September 20, 2023 - A trial of a preventive HIV vaccine candidate has begun enrollment in the United States and South Africa. The Phase 1 trial will evaluate a novel vaccine known as VIR-1388 for its safety and ability to induce an HIV-specific immune response in people.
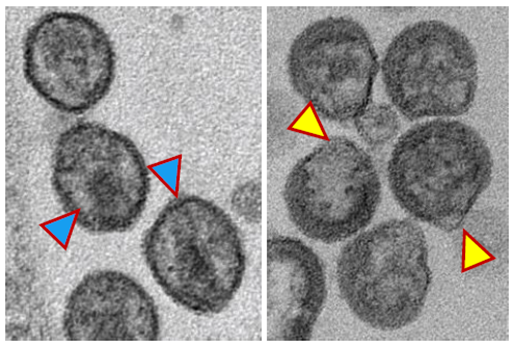
Blocking HIV enzyme reduces infectivity and slows viral rebound
July 26, 2023 - A pair of studies funded by the National Institutes of Health showed that blocking an enzyme involved in forming HIV particles stopped the virus from becoming infectious, suggesting a possible new target for treating HIV infection.
Using content analysis to understand the usage of stigmatizing language in recent scientific literature and its harmful effect on people living with HIV
July 19, 2023 - People living with HIV and experts in the field have long advocated for the use of person-first language, which is a way to emphasize that the disorder, disease, condition, or disability as only one aspect of the whole person. Outdated terms such as “HIV-infected” and “AIDS-infected” are negative and dehumanizing, with the latter being clinically inaccurate. Recently published research supported by NIH performed a content analysis on the usage of HIV-related stigmatizing language in peer-reviewed scientific literature to understand and help address this issue.

Social support promotes HIV suppression among young adults, NIH-funded study suggests
July 17, 2023 - Young adults born with HIV who report average or high levels of social support are more likely to maintain viral suppression than peers with low social support, according to a study of U.S. young adults funded by the NIH. The findings also suggest that having sufficient social support is particularly important just prior to a young adult’s transition from pediatric to adult HIV care.
Daily statin reduces heart disease risk among adults living with HIV
July 24, 2023 – A National Institute of Health-supported study found that statins, a class of cholesterol-lowering medications, may offset the high risk of cardiovascular disease in people living with HIV by more than a third, potentially preventing one in five major cardiovascular events or premature deaths in this population. People living with HIV can have a 50-100% increased risk for cardiovascular disease. The findings are published in the New England Journal of Medicine.

Encouraging First-in-Human Results for a Promising HIV Vaccine
June 6, 2023 – The results of an early phase clinical study, published recently in the journal Science Translational Medicine and earlier in Science, showed that an experimental HIV nanoparticle vaccine is safe in people. While the vaccine alone will not offer HIV protection and is intended to be part of an eventual broader, multistep vaccination regimen, the researchers also determined that it elicited a robust immune response in nearly all 36 healthy adult volunteers.
Daily statin reduces the risk of cardiovascular disease in people living with HIV, large NIH study finds
April 11, 2023 – A National Institutes of Health (NIH) clinical trial ended ahead of schedule because of convincing findings that a daily statin medication could reduce the increased risk of cardiovascular disease among people living with HIV. It was the first large-scale clinical study to test a primary cardiovascular prevention strategy in this population.

HIV Can Persist for Years in Myeloid Cells of People on Antiretroviral Therapy
March 27, 2023 — A subset of white blood cells, known as myeloid cells, can harbor HIV in people who have been virally suppressed for years on antiretroviral therapy, according to findings from a small study supported by the National Institutes of Health.

Mixed-Race Woman Potentially Cured of HIV Using Stem Cell Transplant
March 23, 2023 — A woman with leukemia is likely cured of HIV after receiving a transplant including stem cells from banked umbilical cord blood. The result suggests a way to expand the pool of available stem cells for curing HIV in people who require transplants for other medical conditions.

Learn About the Mpox Vaccine
February 14, 2023 — Demetre Daskalakis, MD, MPH, Director, Division of HIV Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention at CDC and National Mpox Response Deputy Coordinator, answers some of the most common questions about mpox.

Long-acting antiretroviral therapy suppresses HIV among people with unstable housing, mental illnesses, substance use disorders
February 21, 2023 — A long-acting antiretroviral treatment (LA-ART) given every four to eight weeks, and delivered with comprehensive support services, suppressed HIV in people who were previously not virologically suppressed. This is according to an ongoing demonstration study of 133 people with HIV in San Francisco, funded by the National Institutes of Health. The study focused on reaching people who have historically had decreased access to antiretroviral therapy (ART), including people experiencing housing insecurity, mental illnesses, and substance use disorders. The study findings indicate that long-acting injectable ART can benefit people who face many treatment barriers and are historically underserved.
Experimental HIV Vaccine Regimen Safe but Ineffective, Study Finds
January 18, 2023 - An investigational HIV vaccine regimen tested among men who have sex with men (MSM) and transgender people was safe but did not provide protection against HIV acquisition, an independent data and safety monitoring board (DSMB) has determined. The HPX3002/HVTN 706, or “Mosaico,” Phase 3 clinical trial began in 2019 and involved 3,900 volunteers ages 18 to 60 years in Europe, North America, and South America. Based on the DSMB’s recommendation, the study will be discontinued. Participants are being notified of the findings, and further analyses of the study data are planned.

HIV Silencing and Cell Survival Signatures in Infected T Cell Reservoirs
January 5, 2023 — NIH scientists and partners developed technology to provide a genome-wide expression profile of cells harboring latent HIV, which could enable the search for new HIV cure strategies that target infected cell reservoirs.
Early HIV Diagnosis and Treatment Important for Better Long-term Health Outcomes
October 21, 2022 — Starting antiretroviral treatment (ART) early in the course of HIV infection when the immune system is stronger results in better long-term health outcomes compared with delaying ART, according to findings presented today at the IDWeek Conference in Washington, D.C. The findings are based on an extended follow-up of participants in the National Institutes of Health-funded Strategic Timing of Antiretroviral Treatment (START) study.

Three-dose Hepatitis B Vaccine Regimen Protects People with HIV
October 20, 2022 — A three-dose course of the hepatitis B vaccine HEPLISAV-B fully protected adults living with HIV who had never been vaccinated against or infected with the hepatitis B virus (HBV), according to study findings presented today at the IDWeek conference in Washington, D.C. The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, sponsors the ongoing Phase 3 ACTG A5379 clinical study.

NIH Announces Additional Funding Awards for Ending the HIV Epidemic Initiative Implementation Research Projects
October 6, 2022 — Last month, as part of their support of the Ending the HIV Epidemic in the U.S. (EHE) initiative, NIH announced 66 awards to institutions participating in the NIH-funded Centers for AIDS Research (CFAR) and the NIMH AIDS Research Centers (ARC) programs. This was the fourth year of NIH investments in EHE-focused research projects. These new awards total $26 million and will support research in 33 of the EHE priority jurisdictions to strengthen research-community collaborations and enhance the implementation science knowledge base needed to end the HIV epidemic.

White House Publishes Federal Implementation Plan for National HIV/AIDS Strategy
August 26, 2022 — The National HIV/AIDS Strategy (NHAS) Federal Implementation Plan reflects the collaborative work of representatives from 10 federal departments and details more than 380 action items they will implement individually and collaboratively. This plan documents federal agencies’ commitments to programs, policies, research, and other activities needed to meet the NHAS goals. These critical activities, which encompass work to for fiscal years 2022–2025, will move our indicators of progress in the right directions.
New Insights into HIV Latent Cells Yield Potential Cure Targets
July 27, 2022 — In a presentation today at AIDS 2022, the 24th International AIDS Conference in Montreal, scientists with the National Institute of Allergy and Infectious Diseases’ (NIAID) Vaccine Research Center (VRC) and their collaborators described how their use of cutting-edge technology revealed new insights into cellular reservoirs of HIV and what those observations could mean for the next steps in HIV cure research. NIAID is part of the National Institutes of Health.
Finding HIV’s ‘Sweet Spot’
July 22, 2022 — An NIH-funded team has found that patterns of sugars at the surface of our own human immune cells affect their vulnerability to HIV infection.

In people with HIV, treating precancerous anal lesions cuts risk of anal cancer by more than half
June 15, 2022 — Nearly all cases of anal cancer are caused by infection with cancer-causing types of HPV.

Creating a one-stop shop for HIV research
June 9, 2022 — access issue of the Journal of Acquired Immunodeficiency Syndrome (JAIDS) will highlight HIV implementation science findings, reported by scientists and implementations teams funded by the National Institutes of Health through the Ending the HIV Epidemic initiative. (JAIDS)

How Lessons From HIV Research Informed COVID-19 Vaccine Trials
May 12, 2022 — A new study shows how community engagement approaches developed by the HIV Vaccine Trials Network strengthened COVID-19 vaccine trials.

Transactional sex, HIV and health among young cisgender men and transgender women who have sex with men in Thailand
April 8, 2022 — Study examines how recent sex work is identified and the HIV risk factors and service needs among Thai cisgender men who have sex with men and transgender women who exchange sex. (Elsevier: Annals of Epidemiology)

NIH launches clinical trial of three mRNA HIV vaccines
March 14, 2022 — Phase 1 study is among first to examine mRNA technology for HIV.

Researchers document third known case of HIV remission involving stem cell transplant
February 15, 2022 — Woman has remained without detectable HIV for 14 months

First HIV Vaccines Administered in Moderna Clinical Trial
January 2022 – Moderna announces the launch of human clinical trials for an experimental HIV vaccine that uses the same kind of mRNA technology found in Moderna’s COVID-19 vaccine. (The Hill)
NIH celebrates FDA approval of long-acting injectable drug for HIV prevention
December 21, 2021 — Approval marks pivotal expansion of HIV prevention options in the United States.
Experimental mRNA HIV vaccine safe, shows promise in animals
December 9, 2021 — NIH scientists developed vaccine platform.
AIDS and Behavior: Volume 25, supplement issue 2, November 2021
November 2021 — Includes 14 articles on Innovations in Methods and Measurement Science on the Social Determinants of HIV. (AIDS and Behavior).
Too many people with HIV fail to achieve durable viral suppression
November 30, 2021 — NIH-funded study estimates global progress toward UNAIDS goal.
NIH researchers identify how two people controlled HIV after stopping treatment
October 29, 2021 — Different mechanisms suppressed the virus in each person.
NIH Awards More than $20 Million to International HIV Database Centers
July 22, 2021 — The National Institutes of Health has renewed grants to seven regional centers that compose the International epidemiology Databases to Evaluate AIDS (IeDEA), awarding $20.8 million in first-year funding. The 15-year-old IeDEA program efficiently advances knowledge about HIV by pooling and analyzing de-identified health data from more than two million people with HIV on five continents to answer research questions that individual studies cannot address. The grants are expected to last five years and to total an estimated $100 million.
NIH-funded study tests “one-stop” mobile clinics to deliver HIV, substance use care
June 9, 2021 — Mobile clinics could serve as an innovative strategy for expanding access to care.
HIV Related Stigma Research as a Priority at the National Institutes of Health
April 22, 2021 — The National Institute of Health (NIH) recognizes that ending the HIV epidemic will require eliminating HIV-related stigma, which continues to be a critical barrier to the uptake of evidence-based HIV interventions, and in this article the authors provide an overview of NIH HIV stigma research and findings. (AIDS and Behavior)
NIH experts call for accelerated research to address concurrent HIV and COVID-19 pandemics
April 8, 2021 — The COVID-19 pandemic is affecting people with or at risk for HIV indirectly and direct.
HIV/AIDS in the Era of COVID-19: A Juxtaposition of Two Pandemics
April 7, 2021 — Effective responses to concurrent COVID-19 and HIV/AIDS pandemics require a novel coordinated and collaborative global effort to accelerate biomedical research and implementation science to operationalize evidence-based interventions expeditiously. (The Journal of Infectious Diseases)
Profile: Fogarty Fellow Dr. Joseph Matovu investigates HIV self-testing in Ugandan fishing community
March/April 2021 – When social network leaders were trained to disseminate HIV self-testing kits, more than 95% were properly used and returned. (NIH Fogarty International Center Global Health Matters Newsletter, Image courtesy of Moses Mayombwe)
Scaling Up Covid-19 Vaccination in Africa – Lessons from the HIV Pandemic
March 31, 2021 — Concerns about access to Covid-19 vaccines in Africa resemble concerns raised about responding to the HIV pandemic in the mid-1990s and early 2000s, when highly active antiretroviral treatment was accessible in high-income countries but limited in African countries. (The New England Journal of Medicine)
Methodological and Measurement Advances in Social Determinants of HIV: View from NIH
March 29, 2021 — Lessons learned from a Request for Applications that called for methodological innovations around the social determinants of HIV and provided a unique opportunity to reflect on the state of the science. (AIDS and Behavior)
Final HIV Prevention & Treatment Research Highlights from CROI 2021 (video)
March 12, 2021— Dr. Carl Dieffenbach, director of the Division of AIDS at NIH’s National Institute of Allergy and Infectious Diseases, and his colleague Dr. Hillary Hoffman, joined HIV.gov for a conversation about the latest HIV prevention and treatment research presented during the 2021 Conference on Retroviruses and Opportunistic Infections. (HIV.gov)
Unique genotypic features of HIV-1 C gp41 membrane proximal external region variants during pregnancy relate to mother-to-child transmission via breastfeeding
January 2021 - Findings from a pilot study on mother-to-child transmission (MTCT) of HIV-1 through breastfeeding raise the possibility for predicting MTCT by breastfeeding based on identifying mothers with high-risk viral populations. (Journal of Clinical Pediatrics and Neonatology)
Antibody Infusions prevent acquisition of some HIV strains, NIH studies find
Tuesday, January 26, 2021 – Results of Antibody-Mediated Prevention Studies funded by the NIH’s National Institute of Allergy and Infectious Diseases will inform development of long-acting antibody-based HIV prevention tools. (Image Credit: NIAID)
To end HIV epidemic, we must address health disparities
February 19, 2021 — Expert report cites unequal progress in Southern U.S. and among marginalized groups.
This page last reviewed on January 8, 2026


