Breakthrough Lenacapavir Trial Builds on Decades of NIH Investment in Basic Science
How deeply moving it was to be part of the standing ovation when Linda-Gail Bekker, MBChB, Ph.D., announced the monumental results of the PURPOSE 1 trial demonstrating that the medication lenacapavir is 100 percent effective in preventing HIV transmission at the 25th International AIDS Conference (AIDS 2024) in Munich, Germany, in July 2024. This remarkable achievement builds on decades-long investments in scientific research that will yield life-changing results for countless people.
Lenacapavir is the first in a new class of antiretrovirals (ARVs) called capsid inhibitors. The promising results announced at AIDS 2024 are a true game-changer in the prevention of HIV and a testament to the value of NIH-supported basic science. If lenacapavir becomes accessible globally, the number of new HIV cases worldwide will be likely to drop significantly. In PURPOSE 1, lenacapavir was delivered as a twice-yearly injection to cisgender women.
The findings highlight the critical importance of investments in basic science—research to understand basic biological functions and mechanisms—in the development of interventions to prevent, treat, and cure HIV. Studies in the basic sciences can take years to translate to effective prevention strategies or therapies, yet the development of lenacapavir exemplifies the latest example of how basic science leads to breakthroughs that have a profound impact on global public health.
The story of lenacapavir begins long ago, with NIH investment aimed at understanding the 3D structure of viral proteins and their interactions within infected cells. Indeed, it was structure-based design studies, by NIH funded-scientists in the 1980s and 1990s that lead to the development of earlier ARV medications, including reverse transcriptase, protease, integrase inhibitors. Continued support of structural studies at NIH, followed by support of the Centers for HIV Structural Biology in the early 2000s enabled scientists to integrate techniques from structural biology, biochemistry, and cell biology to capture in unprecedented detail the three-dimensional structures of HIV proteins and nucleic acids and their interactions with cellular components. This information helped clarify how the different components interact and revealed new approaches to disrupt those interactions.
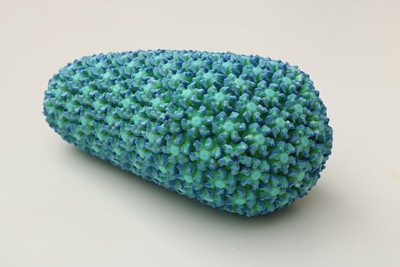
3D-printed model of the HIV capsid, the protein shell that encloses the virus’ genetic material. Credit: NIAID
Capsid emerged as a potential therapeutic target as research demonstrated its importance in the HIV life cycle. The capsid core is a cone-shaped container in the HIV structure that is composed of individual capsid (CA) proteins. The core transports the viral genetic information across the host cell and into the nucleus and protects the virus from the cell’s defense mechanisms, enabling HIV to replicate.
Understanding the structure of the CA protein and how it binds to other CA proteins to create the capsid container was critical to developing a drug that could prevent the capsid core from transporting genetic information. Extensive studies demonstrated that the CA proteins arrange themselves into regular shapes made of six (hexamers) or five (pentamers) CA proteins that make up the lattice of the core structure. Lenacapavir binds two neighboring CA proteins and creates improperly shaped core structures that are unable to enter the nucleus or produce new virus particles. Lenacapavir’s efficacy, even when HIV has mutated to resist other ARVs, reflects the fact that it interferes with multiple steps in the HIV life cycle.

The HIV structure, showing the capsid core, a cone-shaped container in the HIV structure that is composed of individual capsid (CA) proteins. Credit: Adobe Stock
The development of lenacapavir also illustrates an effective collaboration between NIH, academic scientists, and industry. After discovering the capsid’s role in the HIV life cycle, NIH and its grantees collaborated with Gilead Science, Inc., which developed lenacapavir and sponsored the PURPOSE 1 trial. Ongoing research will continue to inform future drug development and clinical research. NIH-funded scientists are conducting research to investigate lenacapavir activity in the body, understand resistance to capsid inhibitors, and develop a method to analyze lenacapavir drug levels. In addition, the HIV Prevention Trials Network is implementing two Gilead-sponsored trials of lenacapavir for PrEP in cisgender women (PURPOSE 3) and people who inject drugs (PURPOSE 4) in the United States.1 Another Gilead-sponsored trial (PURPOSE 2) is studying the efficacy of lenacapavir for PrEP in cisgender men, transgender individuals, and gender nonbinary people who have sex with men in the United States, South America, South Africa, and Asia.
I look forward to learning results of this ongoing research and seeing how we continue to expand on lenacapavir’s success to bring HIV prevention and treatment to more people. Many congratulations to the scientists, partners in academia, and industry for their contributions to the discoveries that led to lenacapavir. Most importantly, our gratitude goes to all those who participated—and who continue to participate—in the research studies.
As my time as Acting OAR Director comes to a close and as a basic scientist, I am thrilled that benefits of fundamental structural studies have come to light with tremendous potential health benefit for people all over the world. Since I arrived at OAR in December 2023, I’ve had a widespread view of the entire NIH HIV research portfolio and have been impressed by the breadth of work that happens within the office. As the coordinator of the NIH HIV research program, OAR works across 22 NIH institutes, centers, and offices to ensure that NIH-supported HIV research is forward-looking, cutting-edge, and responsive to emerging needs. This research leads to scientific advancements and innovative discoveries that have an immeasurable impact in the United States and across the world. It may not always be clear where we will see the next breakthrough, but each new discovery brings us closer to our goal of ending the HIV pandemic.
1 - NIH statement on preliminary efficacy results of twice-yearly lenacapavir for HIV prevention in cisgender women. News release. National Institute of Allergy and Infectious Diseases. June 26, 2024. Accessed September 25, 2024.
https://www.niaid.nih.gov/news-events/nih-statement-preliminary-efficacy-results-twice-yearly-lenacapavir-hiv-prevention
This page last reviewed on July 17, 2025

